Abstract
Background: Chemoimmunotherapy is standard frontline therapy for acute lymphoblastic leukemia (ALL). Rituximab (R) added to hyper-CVAD (HCVAD) has been shown to improve complete remission duration (CRD) and overall survival (OS) in patients (pts) with CD20-positive ALL with ≥20% CD20 expression. Ofatumumab (O) is a type I human antibody that binds to a distinct CD20 epitope compared to rituximab, and induces more potent antibody-dependent and complement mediated cytotoxicity in vitro . We hypothesized that combining ofatumumab with HCVAD may improve outcomes in CD20+ ALL.
Methods: We designed a single-center phase II trial to evaluate the safety and efficacy of O-HCVAD in adults with newly diagnosed ALL with Philadelphia chromosome-negative (Ph-) and CD20+ ≥1%. Patients (pts) received intensive therapy with 4 cycles (cy) of HCVAD [odd cy 1, 3, 5, 7 comprised of fractionated cyclophosphamide, vincristine (VCR), doxorubicin, dexamethasone] alternating with 4 cy of methotrexate-cytarabine (MTX-ara-C, even cy 2, 4, 6, 8). Ofatumumab was administered during cy 1-4 on day 1 and 11 ± 2 of cy 1 and 3; and day 1 and 8 ± 2 of cy 2 and 4. The first dose of ofatumumab was 300 mg and subsequent doses were 2000 mg. Pts received POMP maintenance (6-mercaptopurine, MTX, VCR, prednisone) for nearly 30 months (mo), and intensification with MTX-PEGylated asparaginase on mo 6 and 18 and with O-HCVAD on mo 7 and 19. Intrathecal MTX-ara-C was used for central nervous system prophylaxis. Radiation was used for bulky mediastinal disease if indicated.
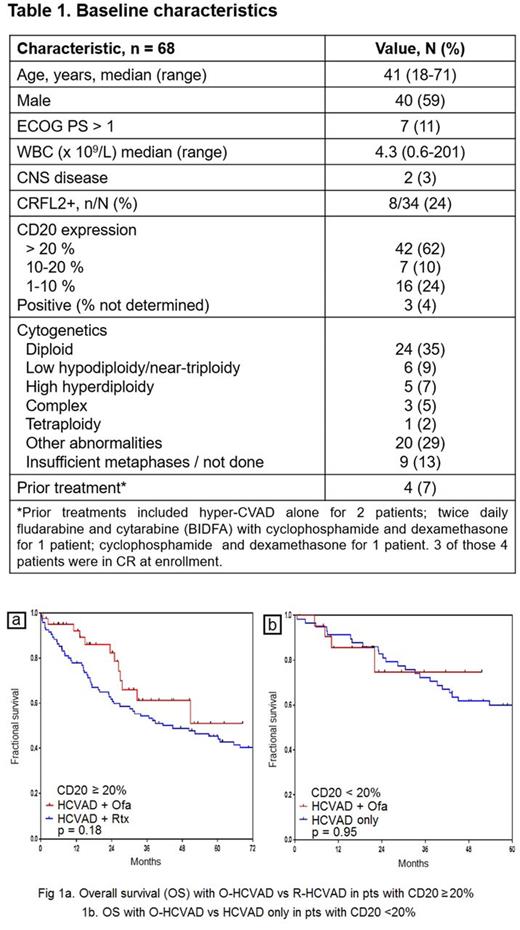
Results: We enrolled 68 pts with Ph- CD20+ ALL between 07/2011 and 05/2017. Four pts had received 1 cy of chemotherapy prior to enrollment and 3 of them were in CR at enrollment. Baseline characteristics are shown in Table 1. Of the 65 pts evaluable for response, 64 pts (98%) achieved CR/CRp. 39/62 (63%) achieved negative minimal residual disease (MRD) at time of CR, and 61/67 pts (91%) achieved negative MRD overall, as assessed by multiparameter flow cytometry. Median time to negative MRD was 0.7 mo (range, 0.4-7.8). Pts received median of 7 intensive phase cy (range, 1-8). Most common non-hematologic grade 3/4 toxicities were infections during consolidation in 75% of pts, infections during induction in 55% pts, and hypokalemia in 51% pts. Median time to recovery of platelets and neutrophils after cy 1 were 21 days (range, 0-60) and 19 days (range, 0-41), respectively. At 22 mo median follow-up, 52 pts (76%) are alive; 17 pts (25%) are receiving maintenance, 15 pts (22%) have relapsed, 12 pts (18%) have completed maintenance, and 11 pts (16%) have received allogeneic stem-cell transplantation (ASCT) in first remission. The 2-year CRD and OS rates were 76% and 81%, respectively. Out of the 16 pts (24%) pts who died, 1 pt had early death from sepsis; 7 pts died after morphologic relapse, 5 pts died in CR/CRp, 1 pt died after MRD+ relapse, 1 pt died after ASCT, and 1 pt died after relapse post-ASCT. Of the pts who died in CR/CRp, 2 pts died from sepsis, 2 pts from therapy-related acute myeloid leukemia, and 1 pt from disseminated adenovirus infection in post-ASCT setting. The 2-yr OS was similar in pts with <20% and ≥20% CD20 expression (75% vs 82%). In pts with CD20 expression ≥20%, there was a trend for better OS favoring O-HCVAD compared to a historical control group treated with R-HCVAD (fig. 1a). In pts with CD20 expression <20%, OS was comparable to a historical control group treated with HCVAD only (fig. 1b).
Conclusions: O-HCVAD is safe, highly effective, and produces durable responses in adults with Ph- CD20+ ALL.
Clinical trial registration information: NCT01363128
Kantarjian: Pfizer: Research Funding; Delta-Fly Pharma: Research Funding; ARIAD: Research Funding; Amgen: Research Funding; Novartis: Research Funding; Bristol-Meyers Squibb: Research Funding. Thomas: Amgen: Honoraria; Pfizer: Honoraria. Cortes: Teva: Research Funding; ImmunoGen: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; ARIAD: Consultancy, Research Funding; Sun Pharma: Research Funding. Jain: Incyte: Research Funding; BMS: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Verastem: Research Funding; Abbvie: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding. Bose: Incyte Corporation: Honoraria. Verstovsek: Bristol Myers Squibb: Research Funding; Incyte: Research Funding; Bristol Myers Squibb: Research Funding; Celgene: Research Funding; Roche: Research Funding; Pfizer: Research Funding; Astrazeneca: Research Funding; Incyte: Research Funding; Seattle Genetics: Research Funding; Blueprint Medicines Corp: Research Funding; Blueprint Medicines Corp: Research Funding; Genentech: Research Funding; Roche: Research Funding; CTI BioPharma Corp: Research Funding; Galena BioPharma: Research Funding; CTI BioPharma Corp: Research Funding; Gilead: Research Funding; Celgene: Research Funding; Promedior: Research Funding; Genentech: Research Funding; Astrazeneca: Research Funding; Galena BioPharma: Research Funding; Gilead: Research Funding; Promedior: Research Funding; NS Pharma: Research Funding; Lilly Oncology: Research Funding; Pfizer: Research Funding; Seattle Genetics: Research Funding; NS Pharma: Research Funding; Lilly Oncology: Research Funding. Daver: Novartis Pharmaceuticals Corporation: Consultancy; Bristol-Myers Squibb Company: Consultancy, Research Funding; Jazz: Consultancy; Pfizer Inc.: Consultancy, Research Funding; Daiichi-Sankyo: Research Funding; Incyte Corporation: Honoraria, Research Funding; Karyopharm: Consultancy, Research Funding; Kiromic: Research Funding; Sunesis Pharmaceuticals, Inc.: Consultancy, Research Funding; Immunogen: Research Funding; Otsuka America Pharmaceutical, Inc.: Consultancy. O'Brien: Pharmacyclics: Consultancy, Other: Research Support: Honorarium, Research Funding; Pfizer: Consultancy, Research Funding; Amgen: Consultancy; Regeneron: Other: Research Support: Honorarium, Research Funding; GSK: Consultancy; ProNAI: Other: Research Support: Honorarium, Research Funding; Acerta: Other: Research Support: Honorarium, Research Funding; CLL Global Research Foundation: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy, Other: Research Support: Honorarium, Research Funding; Vaniam Group LLC: Consultancy; Janssen: Consultancy; Celgene: Consultancy; Aptose Biosciences, Inc.: Consultancy; Alexion: Consultancy; AbbVie: Consultancy; Sunesis: Consultancy; Gilead Sciences, Inc.: Consultancy, Other: Research Support: Honorarium, Research Funding; Astellas: Consultancy. Jabbour: Bristol-Myers Squibb: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal